- Create a new project
File –> new –> project
select Documentum Project and enter a new project name

- Create a new custom type
expand the Documentum Navigator on the left hand side
file–>new–>other–>select Type and click next
enter a new Artifact name and click Finish.
In the main composer window the General tab displays.
1st we need a supertype object. Select dm_document as supertype, because we need the document properties.
2nd on the attributes tab creates a new attribute
In the Structure section, specify the following values for the fields:- Data Type = STRING
- Length = 20

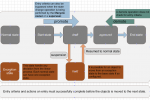
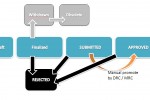
Expand the status attribute node and click on Value mapping. The Conditional Assistance and Value Mapping Table sections appear on the right side. - In the Conditional Assistance section, click on New (screenshot).

Click OK to finish this action.
Save the project and check the Problems and Error log tab. No problems or errors should be displayed. - Display tab:
create a new display configuration (screenshot) In the Configuration Label field, type an unique and comprehensible name. This will be the name of the tab as displayed in Webtop. Select the checkbox Custom attributes only and add all of your custom attributes.
In the Configuration Label field, type an unique and comprehensible name. This will be the name of the tab as displayed in Webtop. Select the checkbox Custom attributes only and add all of your custom attributes.
Click OK to finish this action
In the Application Interface Display section, typean unique and comprehensible name for the Type Label field. This text is displayed in the drop down menu when selecting a type for a new document in Webtop. Save the project and check the Problems and Error log tab. No problems or errors should be displayed.